In a remarkable advancement within the realm of nuclear physics, a dedicated research team at the Institute of Modern Physics (IMP) under the Chinese Academy of Sciences (CAS) has successfully synthesized a new isotope of plutonium, labeled plutonium-227. This significant achievement is documented in a recent publication in the journal Physical Review C. The implications of this discovery extend far beyond mere numbers, prompting critical discussions about the stability and behavior of atomic nuclei, particularly within the context of transuranium elements.
Understanding Shell Closures: The Role of Magic Numbers
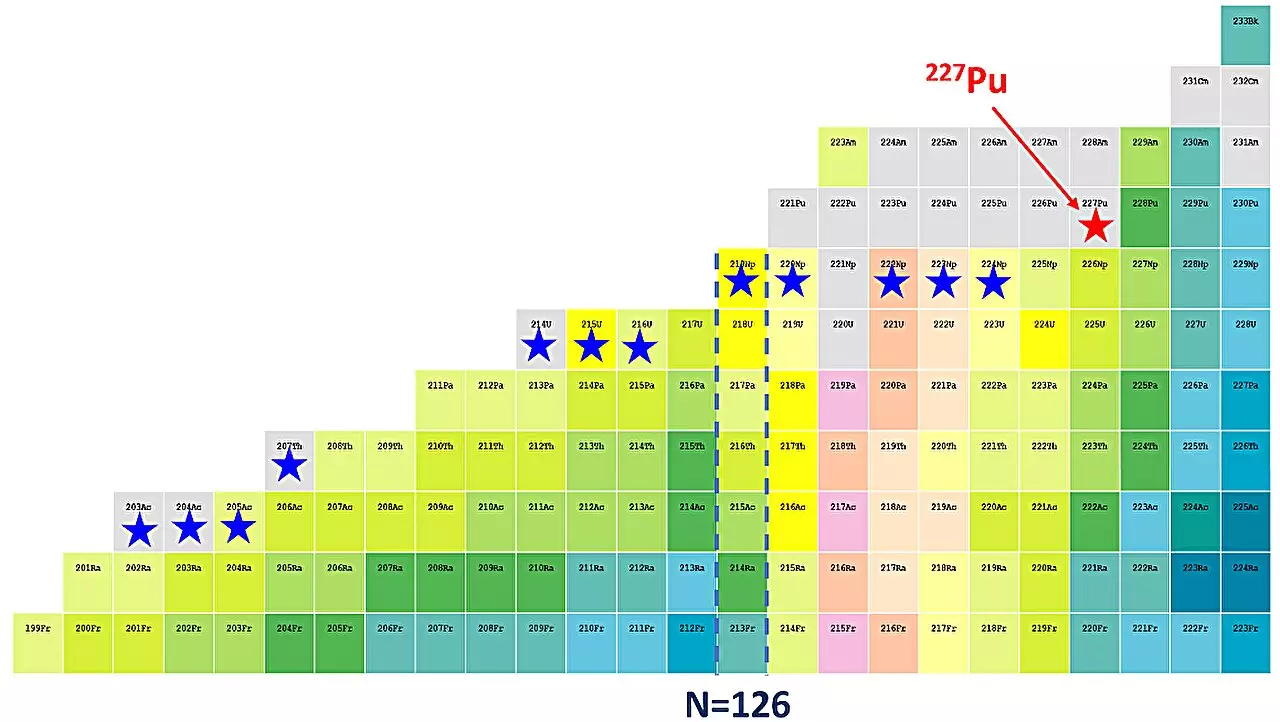
The concept of magic numbers plays a vital role in nuclear physics, relating to specific counts of protons and neutrons that lead to particularly stable configurations of atomic nuclei. These numbers—2, 8, 20, 28, 50, 82, and 126—are indicative of shell closures, akin to the complete shells of electrons that result in noble gas stability. Past investigations have highlighted a concerning trend regarding the waning strength of the neutron shell closure at 126 as one examines heavier elements leading up to uranium. This observation raises crucial questions: how does the shell closure evolve within isotopes beyond uranium, particularly in those of plutonium?
To expand the knowledge of plutonium isotopes and their shell structures, researchers at IMP, alongside collaborators, embarked on a groundbreaking experimental endeavor utilizing the gas-filled recoil separator, known as the Spectrometer for Heavy Atoms and Nuclear Structure. This facility, situated at the Heavy Ion Research Facility in Lanzhou (HIRFL), provided the environment necessary for the sophisticated synthesis of plutonium-227 through a fusion evaporation reaction. The successful creation of this isotope marks the 39th new isotope identified by IMP and bears the distinction of being the first plutonium isotope reported by Chinese scientists.
Through extensive experimentation, the team observed nine decay chains associated with plutonium-227. Their measurements revealed significant characteristics of this newly synthesized isotope, including a β-particle energy of approximately 8191 keV and a half-life of about 0.78 seconds. These data points integrate seamlessly into the established framework of known plutonium isotopes, reinforcing the validity of their experimental approach while also serving as a springboard for future inquiries.
As the research team at IMP contemplates the future trajectory of their exploration into plutonium isotopes, they have identified a clear objective: to deepen the understanding of shell evolution within this complex region of the periodic table. Dr. Yang Huabin, the lead author of the study, emphasizes that while plutonium-227 is notable, it is still seven neutrons shy of reaching the magic number of 126. To unravel the intricacies of shell closures within plutonium, the investigation will undoubtedly shift to lighter isotopes, specifically, the exploration of isotopes ranging from plutonium-221 to plutonium-226.
The synthesis of plutonium-227 stands as a testament to the innovative spirit and methodological rigor of the researchers. As they forge ahead, the findings from this study not only illuminate the nuclear landscape of plutonium but also foster interdisciplinary dialogue regarding the implications of isotopic research in nuclear science and safety.