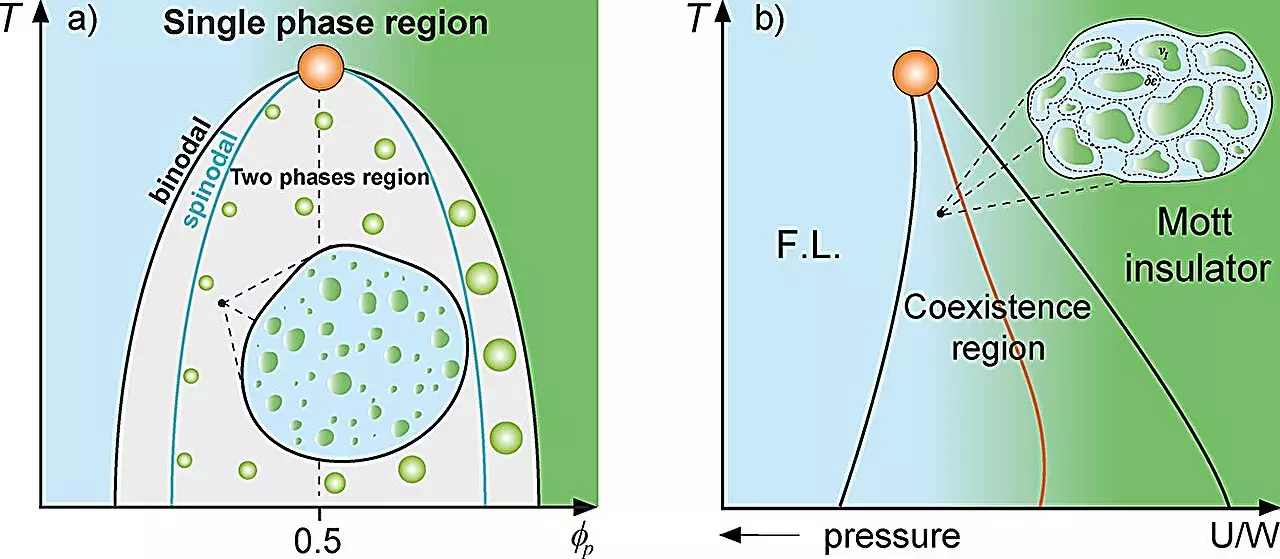
The interaction among various substances is a longstanding domain of study in classical physics, frequently modeled through the lens of mixture theory. This approach allows researchers to understand how different fractions of materials coexist and interact. A compelling example of this concept can be observed in the behavior of supercooled water where high- and low-density phases coexist, or in the phenomenon observed during Mott metal-insulator transitions where metal droplets form within an insulating matrix. Recently, a novel perspective has emerged from Sāo Paulo State University (UNESP) in Brazil, where researchers applied established concepts from condensed matter physics to elucidate the processes surrounding protein compartmentalization within living cells, positing a cellular Griffiths-like phase in analogy with the well-known magnetic Griffiths phase.
Leading this groundbreaking study are Professor Mariano de Souza, a prominent figure in the Institute of Geosciences and Exact Sciences at UNESP, and Ph.D. candidate Lucas Squillante. They propose that cellular dynamics manifest distinct characteristics in response to the localized compartmentalization of proteins—a phenomenon where protein concentration can reach a tipping point, resulting in liquid-liquid phase separation. These separations yield droplet-like compartments vital for cellular function. Professor de Souza succinctly describes the parallels between regions in magnetic systems where magnetized and non-magnetized zones coexist and the rare regions of protein droplet formation within cells.
Using refined thermodynamic models such as the Grüneisen parameter, Flory-Huggins model, and Avramov-Casalini model, the researchers quantitatively demonstrated that cellular dynamics notably slow near the boundaries of phase separation, significantly impacting protein behavior. This decrease in dynamics is crucial, as the emergent Griffiths-like cellular phase may serve as a foundational component of biological development, linking back to theories presented by early biochemists like Aleksandr Oparin. Such theories speculate that the primitive forms of life were predicated upon slow-moving coacervates of organic molecules, offering a primordial scenario conducive to evolutionary processes.
A significant aspect of this study delves into the concept of homochirality—an essential property in biological systems where molecules favor one chiral form over another, leading to the formation of functional biological structures. Professor de Souza articulates how an increase in the diffusion time of proteins paired with diminished stochastic fluctuations might optimize gene expression, marking potential advancements in our understanding of genetic regulation through the lens of physics. This interplay between temporal dynamics and molecular stability in cellular environments offers fresh insights into the baseline mechanisms of life.
The implications of the Griffiths-like phase extend beyond mere theoretical interest. The intersection of liquid-liquid phase separation with disease has become a focal point in medical research, as highlighted by co-author Marcos Minicucci. Here, the ability of proteins to compartmentalize could significantly influence their roles in disease processes, including cancer development. The notion that proteins associated with malignancies may be sequestered within cellular droplets directly aligns with observations concerning tumorigenesis, where such compartmentalization could alter the mutation landscape.
Moreover, disorders such as cataracts and neurodegenerative diseases are implicated in phase separation phenomena, underscoring the medical relevance of this research. In the case of COVID-19, for instance, the coacervation of viral proteins has been linked to immune evasion strategies by the SARS-CoV-2 virus. These examples render the study of phase separation not only a theoretical pursuit but rather a critical component of understanding pathophysiology and potential therapeutic approaches.
This comprehensive study underscores the necessity of interdisciplinary collaboration in science, combining physics, biology, and medicine to unravel the complexities of cellular function. The collective contributions from co-authors across various institutions, including those outside Brazil, such as the University of KwaZulu-Natal and the University of Iceland, reflect a holistic approach to research that transcends individual disciplines.
The Griffiths-like cellular phase presents an exciting avenue for further exploration into protein dynamics, evolutionary biology, and disease management. As scientists deepen their understanding of these complex interactions, they not only shed light on the underlying principles of life itself but also offer promising pathways for addressing some of the most pressing health challenges of our time. Through this synthesis of physics and biology, the potential for significant advancements in both theoretical and applied sciences grows, paving the way for innovative solutions in medicine and beyond.