In the realm of neurological disorders, developments in non-invasive therapeutic methods are crucial. With a significant percentage of patients suffering from drug-resistant epilepsy and recurrent tremors, traditional treatments often fall short. Transcranial focused ultrasound (TFUS) has emerged as a beacon of hope, using high-frequency sound waves to stimulate specific regions of the brain without the need for invasive procedures. Recent advancements in sensor technology have the potential to revolutionize how these treatments are applied, leading to more precise and personalized care.
Recent research led by scientists from Sungkyunkwan University, the Institute for Basic Science, and the Korea Institute of Science and Technology has ushered in a new era for TFUS by developing an innovative sensor. This breakthrough was detailed in a paper published in Nature Electronics, where the team introduced a sensor capable of transforming its shape and tightly adhering to the complex landscape of the brain’s cortical surfaces. According to Donghee Son, the supervising author of the study, prior brain sensors faced significant hurdles in accurately measuring neural signals due to their inability to conform to the brain’s intricate folds. This limitation has often obscured the diagnosis of brain conditions and prevented effective real-time monitoring of brain activities.
Previous models of sensors, such as those engineered by renowned researchers John A. Rogers and Dae-Hyeong Kim, demonstrated enhanced precision in capturing surface-level brain signals. However, they struggled with adherence in areas of significant curvature, which, coupled with micro-movements of the brain and cerebrospinal fluid flow, reduced their long-term efficacy. The vision behind the new sensor was to establish a solution that not only fits snugly in these challenging areas but also maintains consistent contact, thereby offering reliable neural data for extended durations.
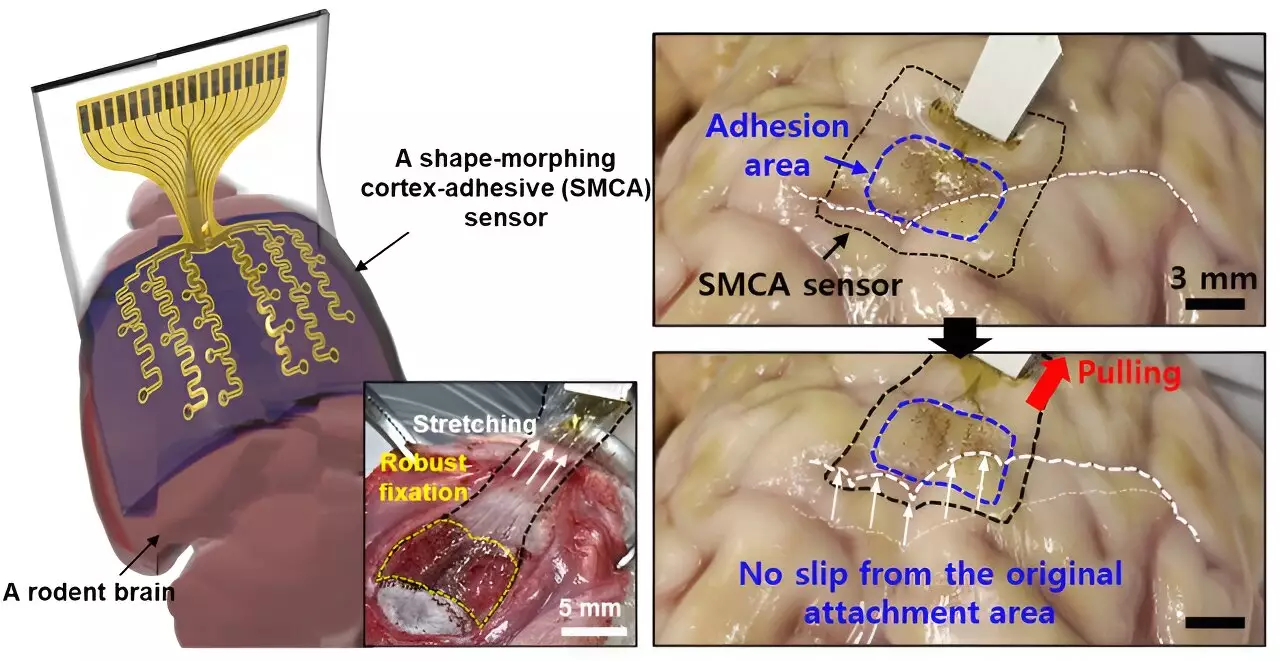
The newly developed sensor, referred to as Electrocorticography (ECoG), features a robust design that securely attaches to brain tissue, significantly reducing noise that could distort signal measurements. This characteristic is monumental for enhancing the efficacy of low-intensity focused ultrasound (LIFU) in epilepsy treatment, which has previously been complicated by inconsistencies between patients. Tailoring treatments to individual brain activity is a pressing need in modern neuroscience, and Son and his team have made critical advancements toward this goal.
The unique structure of the ECoG sensor includes a tri-layer composition: a hydrogel layer that bonds with brain tissue, a shape-morphing polymer layer that adapts to the tissue’s contours, and a stretchable layer integrating gold electrodes. During application, the process begins with the hydrogel forming an immediate bond with the brain, followed by the polymer’s capacity to adjust to the cerebral topography, ensuring that the sensor remains perfectly contoured to the surface and minimizes the risk of voids that could lead to inaccurate readings.
The Promise of Personalized Treatments
As neuroscience researchers continue to explore the prospects of personalized treatments for epilepsy and other neurological disorders, the ability to monitor brain waves in real-time while simultaneously delivering ultrasound stimulation has been largely hindered by traditional sensor noise. The ECoG sensor’s design aims to mitigate these disruptions, thus enabling the user to capture more precise brain wave information. This advancement could open the door to tailored treatment strategies, ensuring that patients receive care that specifically targets their unique brain activity patterns.
Son stressed the significance of this innovation not just for epilepsy management, but for a broader spectrum of neurological conditions. The combination of tissue adherence technology and the sensor’s morphing capabilities symbolize a groundbreaking approach to accurately diagnose and treat disorders that have long remained difficult to understand or manage.
Future Prospects and Expansions
So far, the promising initial tests conducted on awake rodents have verified that this sensor can accurately measure brain waves and modulate seizures effectively. Moving forward, the research group plans to scale the sensor’s capabilities, working towards developing a high-density array that incorporates an increased number of electrode channels. This progression aims to enhance the resolution of brain signal mapping, which is paramount for effective neurological diagnostics and treatments.
Ultimately, the ECoG sensor’s path through clinical trials could pave the way for not only advanced epilepsy treatments but also for future enhancements in brain prosthetics, offering hope to patients with a myriad of neurological challenges. As this technology matures, it could represent a critical evolution in the way we approach brain health and disorders, transforming countless lives through innovative non-invasive therapies.